
When dealing with a range of values (such as the variety of hydrogen ion concentrations encountered in chemistry) that spans many powers of ten, it is convenient to represent them on a more compressed logarithmic scale. Similarly, the concentration of hydrogen ion in a solution made by dissolving 1.0 mol of sodium hydroxide in water will be 1.00 x 10 –14 mol L –1. The concentration of hydroxide ion in such a solution, according to Eq 2, is Hydrochloric acid is a typical strong acid that is totally dissociated in solution:Ī 1.0 M solution of HCl in water therefore does not really contain any significant concentration of HCl molecules at all it is a solution in of H + and Cl – in which the concentrations of both ions are 1.0 mol L –1. Thus, in a neutral solution, both the hydrogen- and hydroxide ion concentrations are 1.00 × 10 –7 mol L –1: The values of these concentrations are constrained by Eq. Take special note of the following definition:Ī neutral solution is one in which the concentrations of H + and OH – ions are identical. This leads to the following important definitions, which you must know: acidic solution This means that H + ions are present in all aqueous solutions, not just acidic ones. The consequences of this are far-reaching, because it implies that if the concentration of H + is large, that of OH – will be small, and vice versa. This expression is known as the ion product of water, and it applies to all aqueous solutions, not just to pure water. Its value varies slightly with temperature, pressure, and the presence of other ions in the solution. The quantity 1.00 x 10 –14is commonly denoted by K w. In which the square brackets refer to the concentrations (in moles per litre) of the substances they enclose. The reason stems from an important relationship that governs the concentrations of H + and OH – ions in aqueous solutions: The degree of dissociation of water is so small that you might wonder why it is even mentioned here. Pure water is practically an insulator, but careful experiments show that even the most highly purified water exhibits a very slight conductivity that corresponds to a concentration of both the H + ion and OH – ions of almost exactly 1.00 × 10 –7mol L –1 at 25☌.Īll chemical reactions that take place in a single phase (such as in a solution) are theoretically "incomplete" and are said to be reversible.

Liquids that contain ions are able to conduct an electric current. Both reactions take place simultaneously, but (1) is so much faster than (2) that only a minute fraction of H 2O molecules are dissociated. This means that in pure water, the reverse reaction, the "dissociation" of water
What's more, this is true even if you start with the purest water attainable. This tendency happens to be very great, so the reaction is practically complete- but not "completely" complete a few stray H + and OH – ions will always be present. The ability of acids to react with bases depends on the tendency of hydrogen ions to combine with hydroxide ions to form water: We end this lesson with a brief discussion of acid-base titration- probably the most frequently carried-out chemistry laboratory operation in the world. But since these topics are intimately dependant on the properties of water and its ability do dissociate into hydrogen and hydroxyl ions, we begin our discussion with this topic.
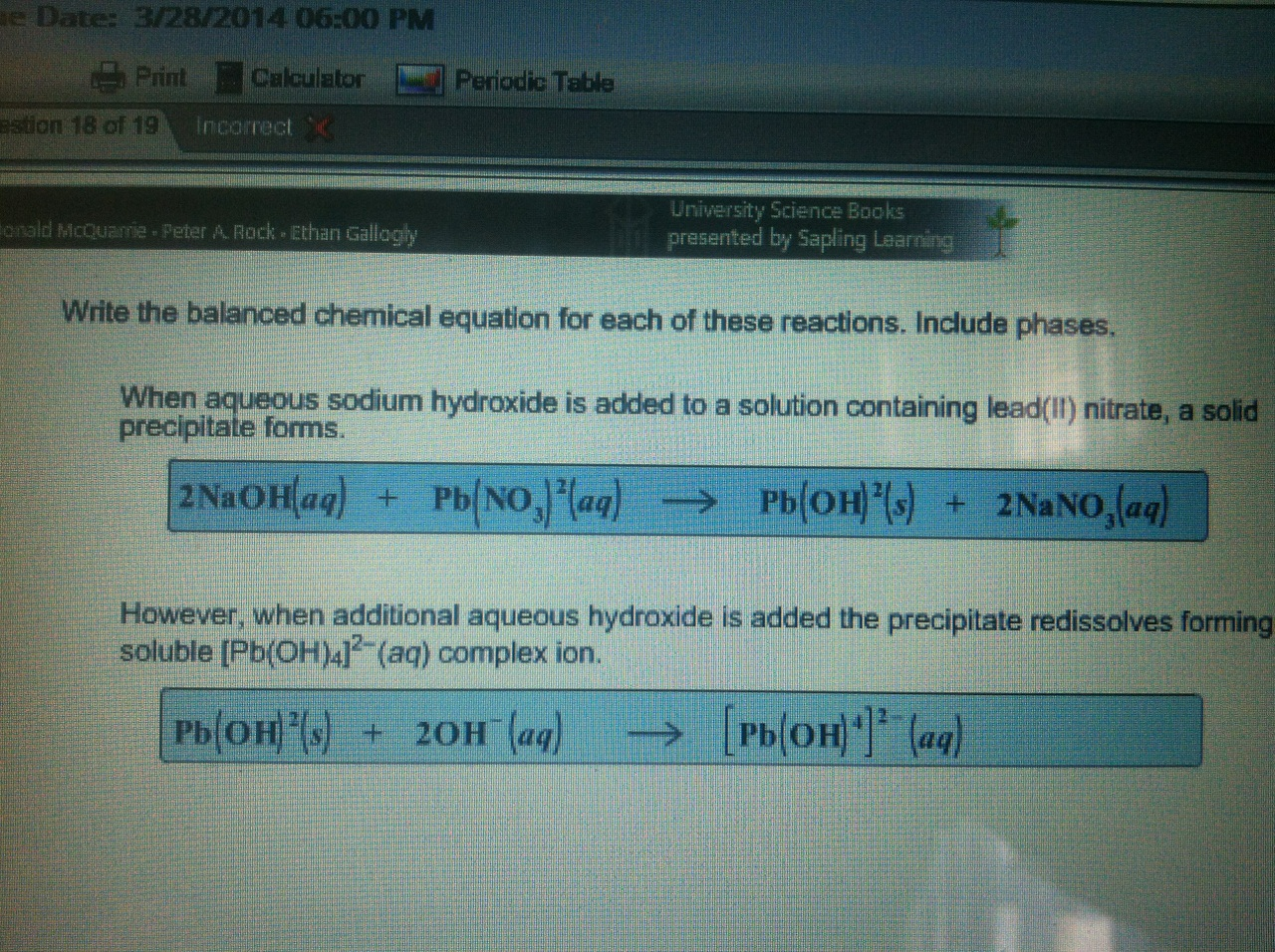
To even those who know very little about chemistry, the term pH is recognized as a measure of "acidity", so the major portion of this unit is devoted to the definition of pH and of the pH scale.


 0 kommentar(er)
0 kommentar(er)
