
H 2SO 4 + AgNO 3 reaction has the following net ionic equation, The process was repeated to obtain three concordant readings.Until the solution changed from being colourless to light pink, the H 2SO 4 from the burette was added dropwise into conical flask.Added 1-2 drops of phenolphthalein indicator.Standardised H 2SO 4 was taken in a burette, and the AgNO 3 was taken in an Erlenmeyer flask.IndicatorĪn acid-base indicator called phenolphthalein is employed to detect the titration’s end point. In H 2SO 4 + AgNO 3 titration, sulphuric acid is used as the titrant, and the titre is AgNO 3.
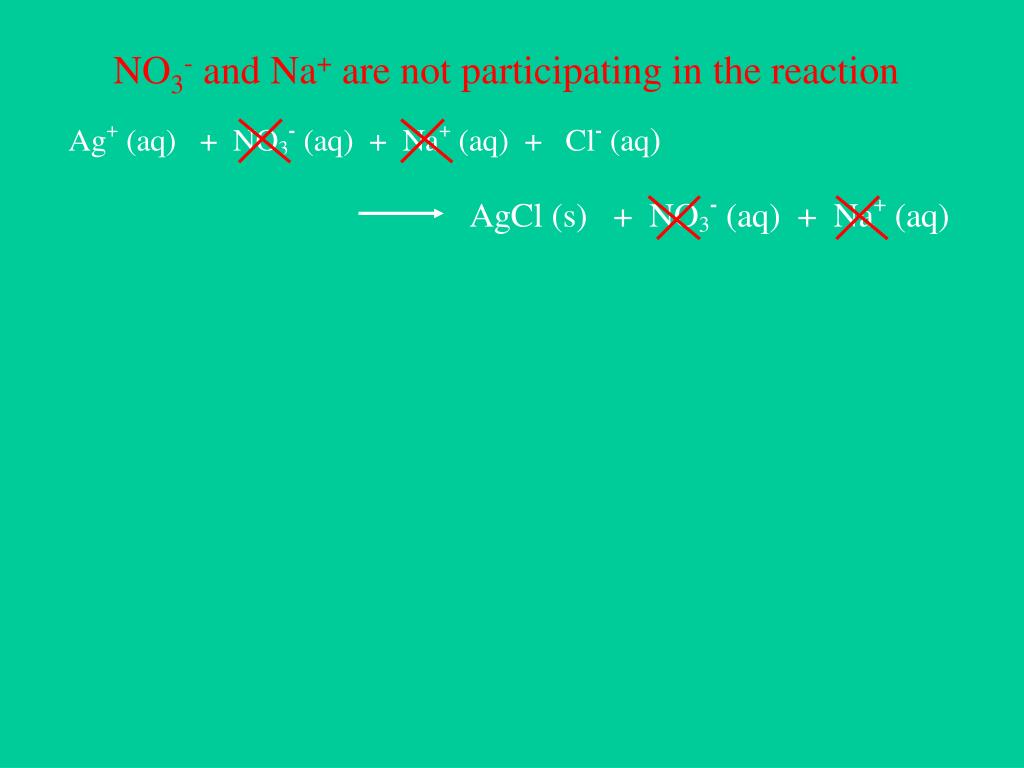
Apparatus usedīurette, pipette, Erlenmeyer flask, burette holder, wash bottle, dropper, volumetric flask and beakers.

However, the reaction should be carried out carefully because the result is a precipitate. We can titrate H 2SO 4 + AgNO 3 to determine the amount of Ag.
#AG 2SO 4 HOW TO#
When AgNO 3 reacts with H 2SO 4, a precipitate of silver sulphate and nitric acid are formed.ĢAgNO 3(aq)+H 2SO 4(aq)→Ag 2SO 4(s)+2HNO 3(aq) How to balance H 2SO 4 + AgNO 3 What is the product of H 2SO 4 and AgNO 3 In this article, we will concentrate on the numerous features of the H 2SO 4 + AgNO 3 reaction in detail. Silver nitrate (AgNO 3) is a precursor for various silver compounds, including those employed in photography. It is a corrosive liquid that is dense, colourless, and oily. Sulphuric acid, also known as oil of vitriol, is a strong acid that completely ionises in water.

Let us examine the reaction between H 2SO 4 and AgNO 3 in depth. Silver nitrate (AgNO 3) is an inorganic compound with antiseptic properties, that reacts with sulfuric acid (H 2SO 4).


 0 kommentar(er)
0 kommentar(er)
